
Minimize Complexity,
Maximize Versatility
In 2003 Medacta conceived a simple, complete and homogeneous system for knee replacement, ranging from uni-compartmental to totally
constrained (hinge). The GMK Primary System is conceived and designed adopting state-of-the-art solutions respecting the natural anatomy
and kinematics of the knee joint.

GMK Primary is also available with SensiTiN, a ceramic-like coating designed to reduce the release of metal ions from the implant.
The GMK Primary product range, including mobile and fixed bearings, offers different levels of constraint:
7 sizes
Anatomical: left and right
Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4)
Cemented: 0.5 mm deep pockets
Cementless: Titanium Plasma Spray (MectaGrip) + HA
.jpg)
Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4) + SensiTiN coating

7 sizes
Anatomical: left and right
Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4)
Cemented: 0.5 mm deep pockets
Cementless: Titanium Plasma Spray (MectaGrip) + HA
.jpg)
Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4) + SensiTiN coating

6 sizes
Anatomical: left and right
Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4)
Cemented: 0.5 mm deep pockets
Cementless: Titanium Plasma Spray (MectaGrip) + HA
.jpg)
Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4) + SensiTiN coating

6 sizes
Anatomical: left and right
Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4)
Cemented: 0.5 mm deep pockets
Cementless: Titanium Plasma Spray (MectaGrip) + HA
.jpg)
Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4) + SensiTiN coating

Symmetric
Anterior flare to accommodate patellar tendon
6 sizes
Five levels of thickness (10, 12 ,14, 17, 20 mm)
Machined Ultra High Molecular Weight Polyethylene (UHMWPE ISO 5834-2)

Symmetric, deep dish
Anterior flare to accommodate patellar tendon
6 sizes
Five levels of thickness (10, 12 ,14, 17, 20 mm)
Machined Ultra High Molecular Weight Polyethylene (UHMWPE ISO 5834-2)

Symmetric
Anterior flare to accommodate patellar tendon
6 sizes
Five levels of thickness (10, 12 ,14, 17, 20 mm)
Additional fixation screw
Machined Ultra High Molecular Weight Polyethylene (UHMWPE ISO 5834-2

Symmetric
Anterior flare to accommodate patellar tendon
7 sizes
Five levels of thickness (10, 12 ,14, 17, 20 mm)
Machined Ultra High Molecular Weight Polyethylene (UHMWPE ISO 5834-2)

Symmetric, deep dish
Anterior flare to accommodate patellar tendon
7 sizes
Five levels of thickness (10, 12 ,14, 17, 20 mm)
Machined Ultra High Molecular Weight Polyethylene (UHMWPE ISO 5834-2)

Anatomical shape
4 sizes
Machined Ultra High Molecular Weight Polyethylene (UHMWPE ISO 5834-2)
Cemented
Three fixation pegs

Round shape
4 sizes
Machined Ultra High Molecular Weight Polyethylene (UHMWPE ISO 5834-2)
Cemented
One central fixation peg

Diameter x length: 11x 30 mm and 11 x 65 mm
Cemented


J-curved sagittal profile helps with more natural knee kinematics, improves knee flexion and promotes femoral roll back. [7,8]

The 4° sloped anterior cut creates a wedge effect that improves primary stability and reduces the risk of anterior notching.

The GMK Primary patello-femoral joint is designed to mimic the natural anatomy and kinematics of the healthy knee in order to optimise patella tracking, improve articular stability and reduce polyethylene wear. GMK incorporates state of the art design features:
MATERIAL
Published papers show that polyethylene that does not undergo any irradiation or thermal treatments, that may affect mechanical properties, may show reduced potential of delamination[1]. Medacta provides machined, non-irradiated polyethylene for all GMK tibial inserts.
ASYMMETRIC SHAPE
Asymmetric design of the tibial baseplate maximises bone coverage, thus assuring optimal load distribution and avoiding risk of overhanging. [14]

MIRROR POLISHED SURFACE
Both for mobile and fixed bearing, the internal surface of the tibial baseplate is mirror polished, minimising the risk of backside wear. [15]

Cementless femoral components are made of Cobalt Chrome alloy (CoCrMo, ISO 5832-4) with a state-of-the-art double coating: plasma sprayed unalloyed titanium (ASTM F1580) plus hydroxyapatite (ASTM F1185). No additional screws are required. [16]

ADAPTING TO YOUR OWN SURGICAL TECHNIQUE
Every surgeon is unique. The GMK instrumentation is specifically designed to provide flexible solutions according to every single surgeon’s preferences: accuracy, ease of use and reproducibility are the guidelines followed for GMK instrumentation development.
The GMK Primary can be implanted following different surgical techniques, according to the surgeon’s philosophy:
Each surgical technique also provides various options, each one designed to fit every surgical scenario :
DESIGN RATIONALE
[1] Anderson MJ, Becker DL, Kieckbusch T. - Patellofemoral complications after posterior-stabilized total knee arthroplasty: a comparison of 2 different implant designs. - J Arthroplasty. 2002 Jun;17(4):422-6
[2] D'Lima DD, Chen PC, Kester MA, Colwell CW Jr. - Impact of patellofemoral design on patellofemoral forces and polyethylene stresses. - J Bone Joint Surg Am. 2003;85-A Suppl 4:85-93.
[3] Morra EA, Greenwald AS. - Patellofemoral replacement polymer stress during daily activities: a finite element study - J Bone Joint Surg Am. 2006 Dec;88 Suppl 4:213-6
[4] Sharma A, Komistek RD, Ranawat CS, Dennis DA, Mahfouz MR - In vivo contact pressures in total knee arthroplasty. - J Arthroplasty. 2007 Apr;22(3):404-16
[5] Baldwin JL, House CK. - Anatomic dimensions of the patella measured during total knee arthroplasty. - J Arthroplasty. 2005 Feb;20(2):250-7.
[6] Kulkarni SK, Freeman MA, Poal-Manresa JC, Asencio JI, Rodriguez JJ. - The patellofemoral joint in total knee arthroplasty: is the design of the trochlea the critical factor? - J Arthroplasty. 2000 Jun;15(4):424-9.
[7] Ranawatt et al, Design may be counterproductive for optimizing flexion after TKR – Clin Orthop Relat Res. 2003 Nov;(416):174-6
[8] Kim et al, Range of motion of standard and high-flexion posterior cruciate-retaining total knee prostheses a prospective randomized study. – J Bone Joint Surg Am. 2009 Aug;91(8):1874-81
[9] Kondo et al. “Arthroscopy for evaluation of polyethylene wear after total knee arthroplasty”, J Orthop Sci, 13:433-437, 2008. Orthop Sci, 13:433-437, 2008
[10] White et al., “Effects of sterilization on wear in total knee arthroplasty”, Clin Orthop, 331:164-71, 1996.
[11] Ries M D, “Highly Cross-Linked Polyethylene. The Debate is Over-In Opposition”, The Journal of Arthroplasty, 20:59-62, 2005.
[12] Baker et al., “The effects of degree of Crosslinking on the fatigue crack initiation and propagation resistane of orthopedic-grade polyethylene”, J Biomed Mater Res A, 66(1):146-54, 2002.
[13] Muratoglu et al., “Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE)”, Biomaterials, 20:1463-70, 1999.
[14] Westrich, Insall, Resection specimen analysis of proximal tibial anatomy based on 100 total knee arthroplasty specimens – J Arth, 1995 Feb;(1);47-51
[15] Engh et al, In vivo deterioration of tibial baseplate locking mechanisms in contemporary modular total knee components. – JBJS Am. 2001 Nov; 83-A(11); 1660-5
[16] Onsten et alt. Hydroxypatite augmentation of the porous coating improves fixation of tibial components, J Bone Joint Surg [Br] 1998;80-B:417-25.
[17] Polyethylene in TKA: Do we really need cross-linked polyethylene?, MORE Journal Vol. 1 May 2011
[18] No differences in human knee morphometry and gender-specific clinical outcomes: a literature review, MORE Journal Vol. 1 May 2011
CLINICAL FOLLOW UP
[19] International Evaluation Group , GMK Primary 1 year clinical follow up – study report , MORE Journal Vol. 1 May 2011
[20] Prim Dr W Anderl, Dr S Dittrich, Mag B Laky, PhD, Dr P Viè, GMK Primary 3 year clinical follow up – study report
[21] Dr P Lambert, Dr V Leon, Dr R Mendelin, Dr E Rinciari, Mechanical Axis Alignment After Total Knee Replacement Performed Through A Navigation System (iMNS) – study report, MORE Journal Vol. 1 May 2011
SINERGY
[22] MyKnee Publication Review MORE Journal Vol. 2 Supplement June 2012
[23] Schroer et al. Mini-subvastus approach for total knee arthroplasty. J of Arthroplasty. 2008 Jan; 23(1): 19-25.
[24] McAllister et al. of minimally invasive surgical techniques on early range of motion after primary totalMcAllister et al. The impact of minimally invasive surgical techniques on early range of motion after primary total knee arthroplasty. J of Arthroplasty. 2008 Jan; 23(1): 10-18.
[25] Bonutti et al. Minimally invasive total knee arthroplasty. JBJS Am. 2004; 86: 26-32.
[26] King et al. Minimally invasive total knee arthroplasty compared with traditional total knee arthroplasty. Assessment of the learning curve and the postoperative recuperative period. JBJS Am. 2007 Jul; 89(7): 1497-503.
[27] Schroer et alt. Isokinetic Strength Testing of Minimally Invasive Total Knee Arthroplasty Recovery. Journal of Arthroplasty, 2010 Feb; 25(2); 274-279
Medacta offers you the opportunity to experience an anatomic knee replacement system with the enhanced accuracy and proven efficiency of a system specifically designed for each individual patient: this is the synergy between GMK system and MyKnee.
MyKnee is a patient-specific cutting block, allowing the surgeon to realize his pre-operative 3D planning, based on CT or MRI images of the patient’s knee.

The MyKnee technology provides a unique set of potential benefits:
Moreover, thanks to MyKnee the surgeon and the hospital can benefit of:
In daily practice, every surgeon faces the increasing demand for excellent and reproducible knee arthroplasty outcomes.
Medacta is committed to developing high level technologies to improve the surgeon’s daily activities and maximize patient satisfaction.
Published papers show how muscle sparing knee Surgery (MSS) minimizes surgical trauma and reduce knee pain, providing earlier functional recovery:
Different MSS approaches are available for TKR., but only the mini subvastus provides a specific set of potential benefits:
Moreover, patients can benefits of mini-subvastus approach not only in the short term:
“At 1 year, the quadriceps strength of the mini-Subvastus TKA knee was equal to that of the uninvolved side, whereas MMP [the medial parapatellar] TKA was 24% weaker than the uninvolved side.” [27]
QUADRICEPS STRENTH COMPARED TO NON-OP LEG AT 1 YEAR
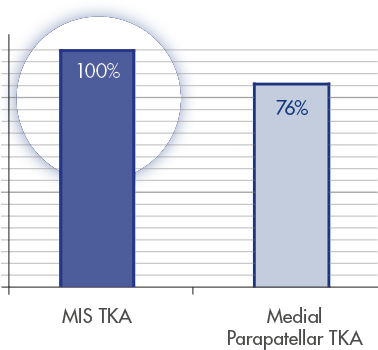
Adapted from Schroer et alt. [27]